Research
Overall Research Objectives
Our research focuses on the use of hematopoietic stem cells and gene therapy for the treatment of degenerative multi-systemic disorders and hereditary nephropathies, with cystinosis as our model. We are developing a multi-systemic strategy involving the transplantation of autologous hematopoietic stem cells genetically modified ex vivo using lentivirus vectors or CRISPR/Cas9 gene editing. The primary objective of the laboratory is the clinical translation of these strategies. Additionally, we are investigating the mechanisms of tissue repair by bone marrow-derived stem cells following hematopoietic stem cell transplantation in the context of a non-hematopoietic disease.
Summary of Research Projects
Hematopoietic Stem Cell Gene Therapy for Cystinosis
 Cystinosis is an autosomal recessive metabolic disease belonging to the family of lysosomal storage disorders. Mutations in the CTNS gene, which encodes a lysosomal cystine transporter, lead to cystine accumulation and multi-organ failure, including end-stage renal failure, blindness, myopathy, diabetes, and central nervous system defects. Treatment is available with the drug cysteamine, which reduces intracellular cystine content, but it only delays the progression of the disease. We showed that wild-type Hematopoietic Stem and Progenitor Cell (HSPC) transplantation in the mouse model of cystinosis, the Ctns^-/- mice, led to abundant tissue integration of bone marrow-derived cells, a significant decrease in tissue cystine accumulation, and long-term preservation of kidney, eye, and thyroid function and structure. Because allogeneic transplants are associated with high risks of mortality and morbidity, we developed an autologous transplantation strategy using HSPCs genetically modified ex vivo to express a functional CTNS gene via a SIN-lentivirus vector. This approach led to a reduction in cystine levels in all tissues and an improvement in kidney function in the Ctns^-/- mice. We conducted toxicology and pharmacology studies, manufacturing development for this strategy, designed the clinical trial, and obtained FDA clearance after submitting an Investigational New Drug (IND) application in December 2018 (ClinicalTrials.gov Identifier #NCT03897361). We have now fully enrolled the Phase 1/2 clinical trial conducted at UC San Diego, with promising clinical outcomes. This program was acquired by Novartis in 2023 for the next phase of the study.
Cystinosis is an autosomal recessive metabolic disease belonging to the family of lysosomal storage disorders. Mutations in the CTNS gene, which encodes a lysosomal cystine transporter, lead to cystine accumulation and multi-organ failure, including end-stage renal failure, blindness, myopathy, diabetes, and central nervous system defects. Treatment is available with the drug cysteamine, which reduces intracellular cystine content, but it only delays the progression of the disease. We showed that wild-type Hematopoietic Stem and Progenitor Cell (HSPC) transplantation in the mouse model of cystinosis, the Ctns^-/- mice, led to abundant tissue integration of bone marrow-derived cells, a significant decrease in tissue cystine accumulation, and long-term preservation of kidney, eye, and thyroid function and structure. Because allogeneic transplants are associated with high risks of mortality and morbidity, we developed an autologous transplantation strategy using HSPCs genetically modified ex vivo to express a functional CTNS gene via a SIN-lentivirus vector. This approach led to a reduction in cystine levels in all tissues and an improvement in kidney function in the Ctns^-/- mice. We conducted toxicology and pharmacology studies, manufacturing development for this strategy, designed the clinical trial, and obtained FDA clearance after submitting an Investigational New Drug (IND) application in December 2018 (ClinicalTrials.gov Identifier #NCT03897361). We have now fully enrolled the Phase 1/2 clinical trial conducted at UC San Diego, with promising clinical outcomes. This program was acquired by Novartis in 2023 for the next phase of the study.Mechanism of Hematopoietic Stem Cell-Mediated Therapy in Cystinosis
 The extent of efficacy of HSPCs to rescue cystinosis in the Ctns^-/- mice was surprising, especially considering that cystinosin is a transmembrane lysosomal protein. To address the mechanism of action, we showed that most of the HSPCs differentiated into macrophages, which generated long tubular extensions known as tunneling nanotubes (TNTs), capable of mediating the transfer of cystinosin-bearing lysosomes into deficient host cells. We also demonstrated, for the first time, that TNTs could cross the renal tubular basement membrane in vivo and transfer cystinosin-bearing lysosomes to proximal tubular cells, providing a mechanism underlying long-term kidney preservation after HSPC transplantation in the Ctns^-/- mice. While cross-correction has already been demonstrated in several lysosomal storage disorders caused by defective soluble lysosomal enzymes through secretion-recapture or enzyme replacement therapy, our study is the first demonstration of cross-correction in the context of a lysosomal transmembrane protein, creating the concept of lysosomal cross-correction. We demonstrated the same mechanism in the eye and thyroid. We further characterized the macrophages capable of forming TNTs and showed that cystinotic cells enhanced the formation of TNTs and the transfer of lysosomes and mitochondria. Understanding the formation and mechanism of action of TNTs holds significant potential for advancing regenerative medicine.
The extent of efficacy of HSPCs to rescue cystinosis in the Ctns^-/- mice was surprising, especially considering that cystinosin is a transmembrane lysosomal protein. To address the mechanism of action, we showed that most of the HSPCs differentiated into macrophages, which generated long tubular extensions known as tunneling nanotubes (TNTs), capable of mediating the transfer of cystinosin-bearing lysosomes into deficient host cells. We also demonstrated, for the first time, that TNTs could cross the renal tubular basement membrane in vivo and transfer cystinosin-bearing lysosomes to proximal tubular cells, providing a mechanism underlying long-term kidney preservation after HSPC transplantation in the Ctns^-/- mice. While cross-correction has already been demonstrated in several lysosomal storage disorders caused by defective soluble lysosomal enzymes through secretion-recapture or enzyme replacement therapy, our study is the first demonstration of cross-correction in the context of a lysosomal transmembrane protein, creating the concept of lysosomal cross-correction. We demonstrated the same mechanism in the eye and thyroid. We further characterized the macrophages capable of forming TNTs and showed that cystinotic cells enhanced the formation of TNTs and the transfer of lysosomes and mitochondria. Understanding the formation and mechanism of action of TNTs holds significant potential for advancing regenerative medicine.
Hematopoietic Stem Cell Gene Therapy for Friedreich's Ataxia
 Because mitochondria can also be transferred via tunneling nanotubes, we hypothesized that HSPC transplantation could treat mitochondrial diseases. We tested this hypothesis in a mouse model of Friedreich’s Ataxia (FRDA), the YG8R mice. FRDA is an autosomal recessive mitochondrial disease characterized by neurodegeneration, cardiomyopathy, and muscle weakness, with patients typically becoming wheelchair-bound within 10-15 years of onset. FRDA is caused by a homozygous GAA repeat expansion mutation within intron 1 of the frataxin gene (FXN), leading to reduced expression of the mitochondrial protein frataxin. There is currently no effective treatment for FRDA. We showed that HSPC transplantation in the YG8R mice completely prevented the development of locomotor deficits and muscle weakness. Degeneration of the large sensory neurons in the dorsal root ganglia (DRG) was also prevented in the treated mice, along with mitochondrial dysfunction in the brain, skeletal muscle, and heart. Abundant GFP+ HSPC-derived cells were observed in tissues as differentiated phagocytic cells, such as microglia in the brain and spinal cord, and macrophages in the DRGs, heart, and skeletal muscle. Finally, we observed the in vivo transfer of frataxin-GFP and COX8-GFP mitochondrial proteins from HSPC-derived microglia/macrophages to diseased neurons and cardiac/muscle myocytes. Thus, this work shows for the first time that single HSPC transplantation holds the potential to become a lifelong curative therapy for FRDA that may prevent the sequelae of this disorder. Our objective is to develop an autologous HSPC gene therapy for FRDA. Because the GAA repeat mutation is in an intron and is carried by 98% of patients with FRDA, we developed a strategy to remove the hyper-expansion mutation using CRISPR/Cas9 technology and optimized the protocol for human CD34+ cells isolated from FRDA patients. The gene-corrected cells exhibit increased frataxin expression and improved mitochondrial function. Our goal is the translational development of this new cell and gene therapy approach for FRDA, a disorder for which there is a pressing unmet medical need. Moreover, this study may offer new perspectives in the treatment of other diseases involving mitochondrial genetic defects.
Because mitochondria can also be transferred via tunneling nanotubes, we hypothesized that HSPC transplantation could treat mitochondrial diseases. We tested this hypothesis in a mouse model of Friedreich’s Ataxia (FRDA), the YG8R mice. FRDA is an autosomal recessive mitochondrial disease characterized by neurodegeneration, cardiomyopathy, and muscle weakness, with patients typically becoming wheelchair-bound within 10-15 years of onset. FRDA is caused by a homozygous GAA repeat expansion mutation within intron 1 of the frataxin gene (FXN), leading to reduced expression of the mitochondrial protein frataxin. There is currently no effective treatment for FRDA. We showed that HSPC transplantation in the YG8R mice completely prevented the development of locomotor deficits and muscle weakness. Degeneration of the large sensory neurons in the dorsal root ganglia (DRG) was also prevented in the treated mice, along with mitochondrial dysfunction in the brain, skeletal muscle, and heart. Abundant GFP+ HSPC-derived cells were observed in tissues as differentiated phagocytic cells, such as microglia in the brain and spinal cord, and macrophages in the DRGs, heart, and skeletal muscle. Finally, we observed the in vivo transfer of frataxin-GFP and COX8-GFP mitochondrial proteins from HSPC-derived microglia/macrophages to diseased neurons and cardiac/muscle myocytes. Thus, this work shows for the first time that single HSPC transplantation holds the potential to become a lifelong curative therapy for FRDA that may prevent the sequelae of this disorder. Our objective is to develop an autologous HSPC gene therapy for FRDA. Because the GAA repeat mutation is in an intron and is carried by 98% of patients with FRDA, we developed a strategy to remove the hyper-expansion mutation using CRISPR/Cas9 technology and optimized the protocol for human CD34+ cells isolated from FRDA patients. The gene-corrected cells exhibit increased frataxin expression and improved mitochondrial function. Our goal is the translational development of this new cell and gene therapy approach for FRDA, a disorder for which there is a pressing unmet medical need. Moreover, this study may offer new perspectives in the treatment of other diseases involving mitochondrial genetic defects.Hematopoietic Stem Cell Gene Therapy for Alzheimer's Disease
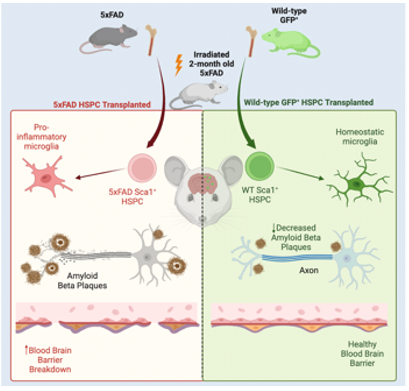
Microglia have been shown to be involved in the clearance of the Aβ plaque, which is impaired in Alzheimer’s disease (AD). As such, promoting Aβ plaque clearance by healthy microglia provides a potential therapeutic opportunity. Using the 5xFAD double transgenic mouse model of AD, which expresses mutant human APP and PSEN1 genes, we demonstrated that a single WT HSPC transplantation led to the preservation of memory and neurocognitive performance, as well as a reduction in the Aβ plaque burden in the hippocampus and cortex. WTHSPCs differentiated into microglia with active amyloid plaque clearance potential while also leading to a reduction in neuroinflammation and microgliosis. This work opens new therapeutic avenues using HSPC gene therapy for the treatment of AD. We are also investigating the mechanisms by which WT HSPCs are able to rescue AD.
Kidney-Targeted Gene Delivery Using AAV
A wide range of monogenic kidney disorders has been identified and so far no gene therapy approach has been developed to target specifically the kidney whereas renal transplantation is associated with significant morbidity and mortality. Moreover, due to the severe shortage of donor organs, patients may wait three to six years for transplantation. The main goal of our project is to develop an efficient and minimally invasive kidney-targeted gene delivery system using recombinant Adeno-Associated Viruses (rAAV). We optimized a kidney-targeted gene delivery via retrograde renal vein injection by testing several rAAV serotypes that have the potential of transducing a wide range of renal cells and showed that the serotype 9 was the most efficient to transduce the different type of renal cells. This strategy could be used to prevent kidney
transplantation in many monogenic hereditary nephropathies.
Hematopoietic Stem and Progenitor Cell Gene Therapy for Mucopolysaccharidosis Type IIIC
Mucopolysaccharidosis type IIIC (MPSIIIC), or Sanfilippo Syndrome Type C, is a severe lysosomal storage disorder caused by loss-of-function mutations in the HGSNAT gene, which encodes the lysosomal transmembrane enzyme heparan acetyl-CoA:alpha-glucosaminide N-acetyltransferase. This enzyme catalyses the N-acetylation of α-glucosamine residues of heparan sulfate, leading to the accumulation of the glycosaminoglycan (GAG) heparan sulfate (HS) multiple tissues. MPS IIIC is characterized by neurodegenerative disease that eventually leads to loss of vision, speech, and motor function. Currently, there is no effective treatment for MPS IIIC. Based on our work in cystinosis, we tested whether wild-type (WT) HSPC transplantation in MPSIIIC mice. WT HSPC transplantation led to a decrease in the MPS IIIC-specific non-reducing end carbohydrate and total heparan sulfate, as well as improvements in neurological defects, reductions in splenomegaly, urine retention and tissue inflammation in Hgsnat^-/- mice. We are now testing a gene modified HSPC approach using a lentiviral vector to modify HSPCs so that they can express a functional HGSNAT. The aim of this study is to develop an autologous gene modified stem cell therapy for MPS IIIC.
