Therapeutics & Vaccines
Novel Antibiotic Discovery

The continual emergence of antibiotic resistance among medically-important bacterial pathogens poses a great challenge to the public health. Sadly, the pipeline of new antibiotics in pharmaceutical development has yet to match this threat, with few novel antibiotic scaffolds developed in the last few decades. Our group is pursuing multiple parallel approaches for novel antibiotic discovery. These include evaluation of new chemical entities generated from marine actinomycete-derived natural product libraries, chemical genomic platforms, virtual screens, and medical chemistry modification of lead compounds. We also believe that outside-the-box approaches to infectious disease therapy including inhibition of virulence factors (e.g. S. aureus pigment) or pharmacological augmentation of phagocytic cell function (e.g. HIF-1α boosting) represent critical areas for exploration. Finally, through an NICHD-sponsored UCSD Research Program in Developmental Pharmacology, we are exploring synergy of pharmaceutical antibiotics with natural antimicrobial peptides, with a goal of optimizing therapy through innate immune sensitization. Collaborators include W. Fenical (SIO/UCSD), P. Dorrestein (UCSD), M. Burkart (UCSD) and E. Capparelli (UCSD).
Cellular Membranes as Sepsis Therapeutics
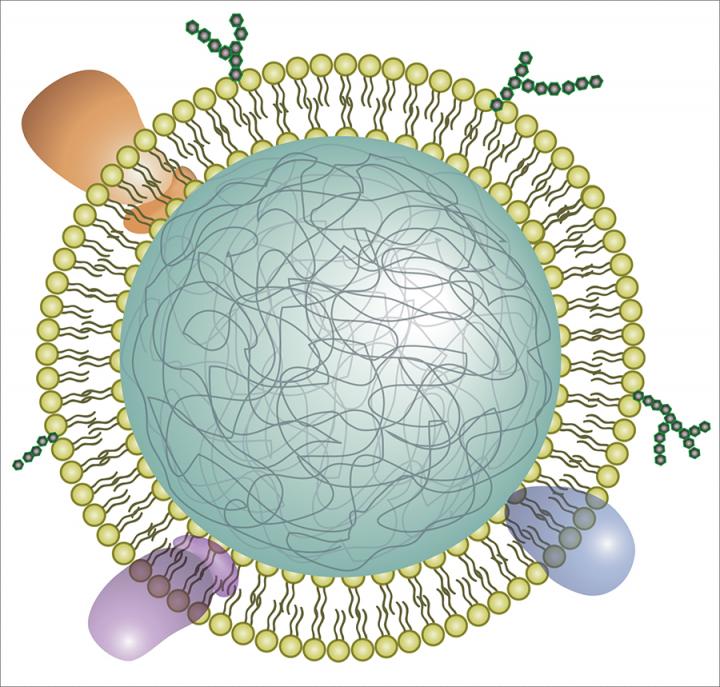
Bacterial sepsis, a life-threatening event involving multiple organ systems due to a improper immune response is a leading cause of human mortality whose successful treatment is increasingly compromised by expanding antibiotic resistance. The complexity of sepsis underlies both a high mortality rate (~49 million annually) and high therapuetic failure rate (>120 clinical candidates have failed). We take a multifactorial approach to treat an incredibly complicated disease, utilizing host-derived cellular membranes to act as biomimetics as a broad spectrum sepsis intervention. Harnassing the natural properties of host membranes, nanopsonges can absorb and neutralize harmful microbial toxins, proinflammatory PAMPs, and uncontrolled cytokines. Other abilities include acting as a decoy for bacterial toxin and auto-antibodies. We are targeting clinically relevant pathogens including MRSA, S. pneumoniae, E coli, and P. aeruginosa. Collaborators include L. Zhang (UCSD) and Cellics Therapeutics Inc.
Bacterial Membrane Derived Nanovaccines
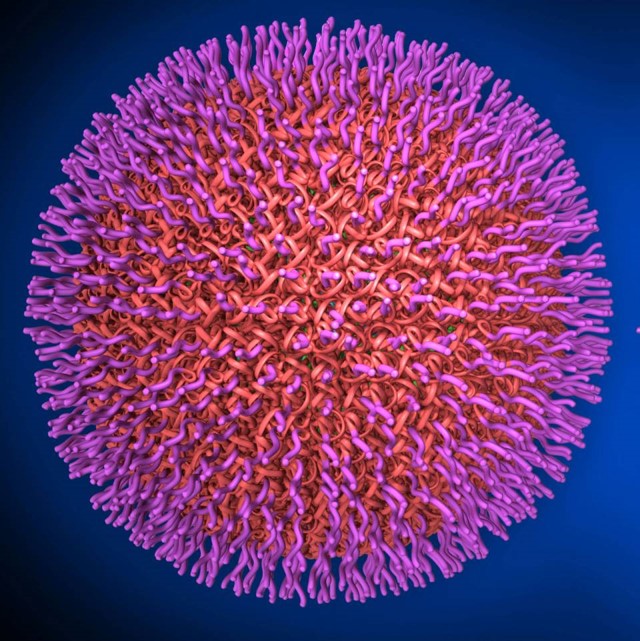
The growing antibiotic resistance crisis and a dearth of new antibiotics in clinical development, alternative solutions to multidrug resistant bacteria are critically needed. We have developed a novel nanoparticle vaccine platform in collaboration with the Zhang Lab in the Department of Nanoengineering (UCSD) in which outer membrane vesicles are utilized as vaccine antigens. Our vaccines currently provide protection in murine models of Acinetobacter baumannii sepsis & pneumonia, Pseudomonas aeruginosa sepsis & pneumonia, as well as E coli sepsis. Ongoing work explores the mechanisms of innate and adaptive synergy following vaccination and is expanding the vaccine repertoire to additional high priority clinical targets including N. gonorrhea, K. pneumoniae, and C. auris. Our collaborators include L. Zhang (UCSD).
Drug Repurposing

The acquisition of resistance by microbial pathogens occurs significantly faster than the antibiotic development pipeline can keep up. An often overlooked fact is that infections are complex host-pathogen interaction in which numerous antimicrobials have already been employed by the innate immune system, long before the patient is treated by a physician. In this arm of our research, we investigate novel approaches that seek to tip the balance back in favor of the host. To this end, we utilize a framework in which medications already FDA approved for other indications and ineffective antibiotics can be combined to synergize with innate immune killing. In this manner, drugs such as Ticagrelor, a drug commonly prescribed after myocardial infarcation, can be repurposed and others such as azithromycin, an antibiotic previously shown to lack activity in standard testing media, can be rediscovered as potent synergizers of the innate immune cells. We also champion the use of biologically relevant testing media in standard microbial inhibitory assays (MICs) as bacteriologic media fails to account for physiological conditions.
Other areas of research:



